SACRAMENTO, Calif. — Kaiser Permanente announced Monday plans to expand its COVID-19 vaccine trials to include children as young as 5 years old.
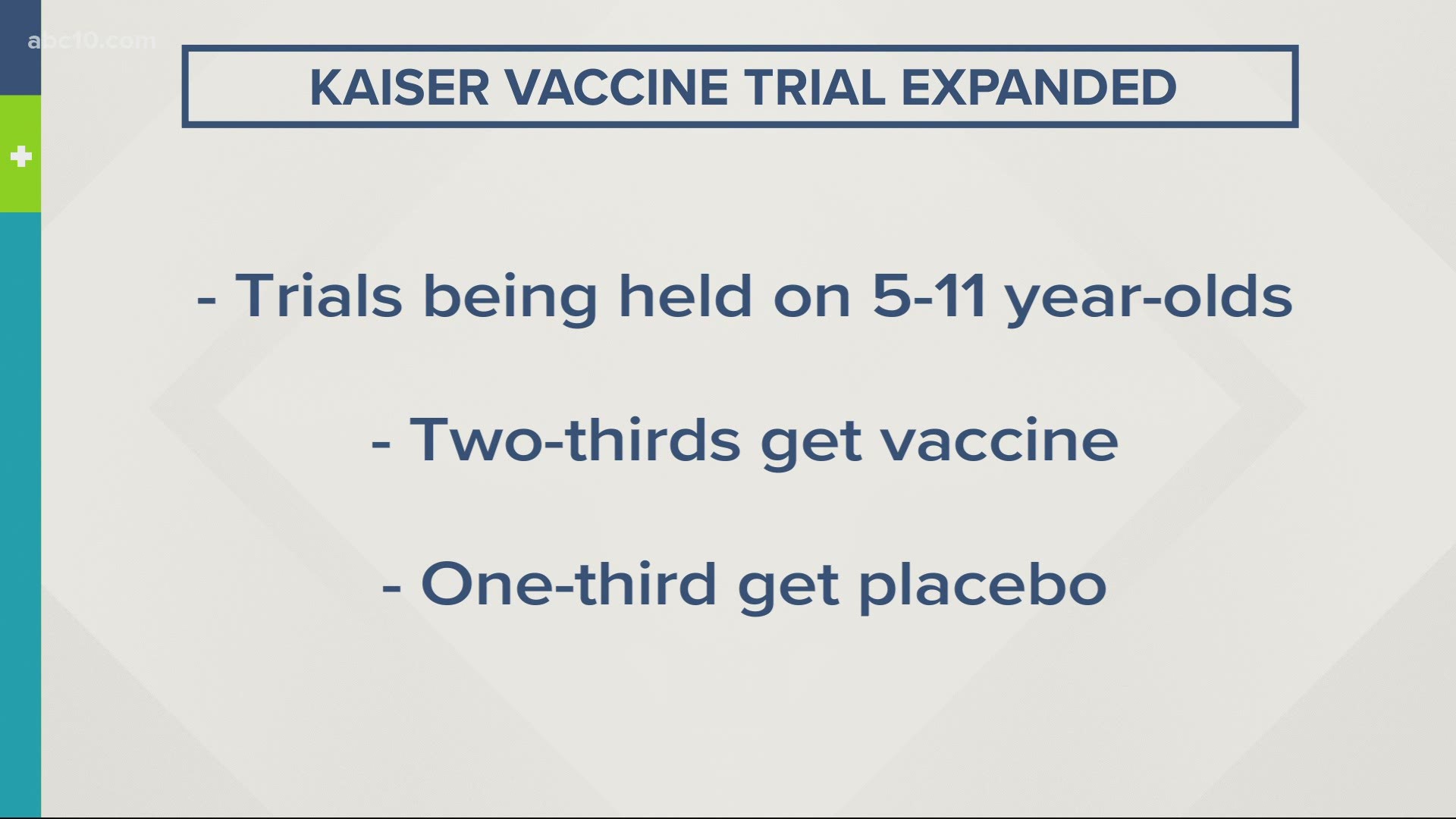
According to a press release, the hospital system is conducting pediatric trials for the Pfizer vaccine in Oakland, Sacramento and Santa Clara. The trials are for children between the ages of 5 and 11 years old.
Millions of adults and children as young as 12 years old have already received the COVID-19 vaccines. As of Monday, June 7, 303 million doses of the vaccine have been administered to Americans, with 140 million considered full vaccinated.
Eleven-year-old Luci Guardino is participating in the trial. Her mother, Dr. Stephanie Yee-Guardino, is a pediatrician at Kaiser Permanente South Sacramento.
“As a physician and a parent, when we found out about the study and we asked our three kids if they wanted to participate, I was really happy that they were interested,” Dr. Yee-Guardino said.
Luci said a big part of her motivation to take part was to get back to the life she remembered before the pandemic.
“It was important for me to take part in the study because I wanted to make sure it was safe for kids my age to get the COVID vaccine,” Luci said before reflecting on what she hopes to gain. "Simple things like going to the movies, eating indoors, having friends over. I definitely wanted to join the study so we can get back to normal."
The pediatric trials are enrolling about 75 children. In the double-blind study, two-thirds of participants will get the vaccine while the remaining third get a placebo. During the trial, data researchers gather data on safety, immune response, and efficacy.
Click here to learn more about the pediatric trials.
"The folks living outside are just trying to survive... they want to stay warm, they want to eat," said Loaves & Fishes Joe Smith. "People have nowhere to go."