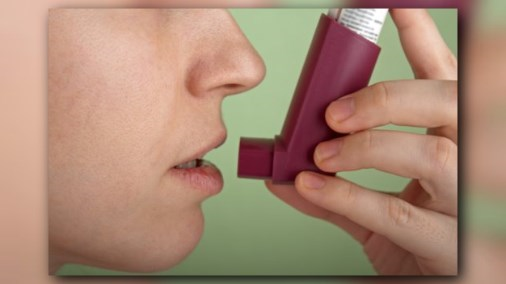
LITTLE ROCK, Ark. (KTHV) -- A nationwide recall has been announced for inhalers produced by GlaxoSmithKline, LLC, also known as GSK.
Almost 600,000 inhalers are being recalled due to a defective delivery system. A report from the FDA said that some inhalers were "out of specification results for leak rate."
The recall was classified as a Class II recall which means "the products might cause a temporary health problem, or pose only a slight threat of a serious nature," according to the FDA.
The inhalers effected by the recall were made in a plant in Zebulon, North Carolina. The recall is reported to be voluntary and was initiated by GSK.
The product is described as: Ventiolin HFA (albuterol sulfate) Inhalation Aerosol, 90 mcg per actuation, 200 metered inhalations, net weight 18 g inhalers, RX only. If you would like to read more on this recall click here.